9+ Basic Chemistry Chapter 2 Answer Key
Molar mass the mass of 1 mole of a substance. C The preceding answers.

Chapter 2 Study Guide Inorganic Chemistry
Chapter 4 introduces economic structuralism via the.

. A systematic approach used in all scientific study. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. By searching the title publisher or authors of guide you really.
Find molar mass by adding together atomic masses of the. AP Statistics Chapters 1-3 Ap statistics chapter 3 test answer key. Key terms with phonetic pronunciations help build vocabulary.
As the bubbles rise the pressure decreases so their volume increases as suggested by Boyles law. File Type PDF Anatomy Chapter 2 Basic Chemistry Packet Answer Key experience. 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 15.
A The number of particles in the gas increases as the volume increases. Vocabulary for the first unit covered in AP statistics. Chemical properties describe the ways in which a given substance undergoes a chemical reaction.
The six elements that are most abundant in the human body are. Chapter 2 discusses the realist theory of IR and its evolution. It will definitely ease you to look guide Chapter 2 Basic Chemistry Packet Answer Key as you such as.
Calculate the molar masses of the following. Com - graded homework questions problems and tutorials. Fill in the blanks from the information provided on the presentation.
1 matter is composed of extremely small particles called atoms 2 Atoms of a given element are identical 3 Different atoms combine in simple whole number ratios to. A type of numerical graph in which data must. This is an example of.
BASIC CHEMISTRY Part 2 Name. And the mass number is 12. Bookmark File PDF Chapter 2 Basic Chemistry Answers book in the text art and problems while placing the chemistry in a biological environmental or geological context.
MasteringChemistry Mastering chemistry answer key chapter 4. The CD-ROM that accompanies the book. Chapter 3 provides a close look to liberal tradition and its reflections in IR.
Carbon oxygen hydrogen nitrogen phosphorus and sulfur Na stands for the element sodium. Matter and Change. A mixture is two or more substances.
Play this game to review Chemistry.

Rd Sharma Solutions For Class 9 Maths Chapter 16 Circles Free Pdf Download

Browse Questions For Chemistry 101

Q2 Use Number Line And Add The Following Integers A 9 6 B 5 11 C 1 7 D 5 10 E 1 2 3 F 2 8 4

Cbse Papers Questions Answers Mcq Class Ix

Pdf Stanley I Sandler Solution Chemical Biochemical And Engineering Thermodynamics Fernanda Maslova Academia Edu

Synthesis And Properties Of The Geometrically Controlled Mixed Valence Tetrahedral Ruii2ruiii2l6 Cluster Cai 2005 European Journal Of Inorganic Chemistry Wiley Online Library

Aplus Blog Std 9 All Subjects First Bell Notes Worksheet
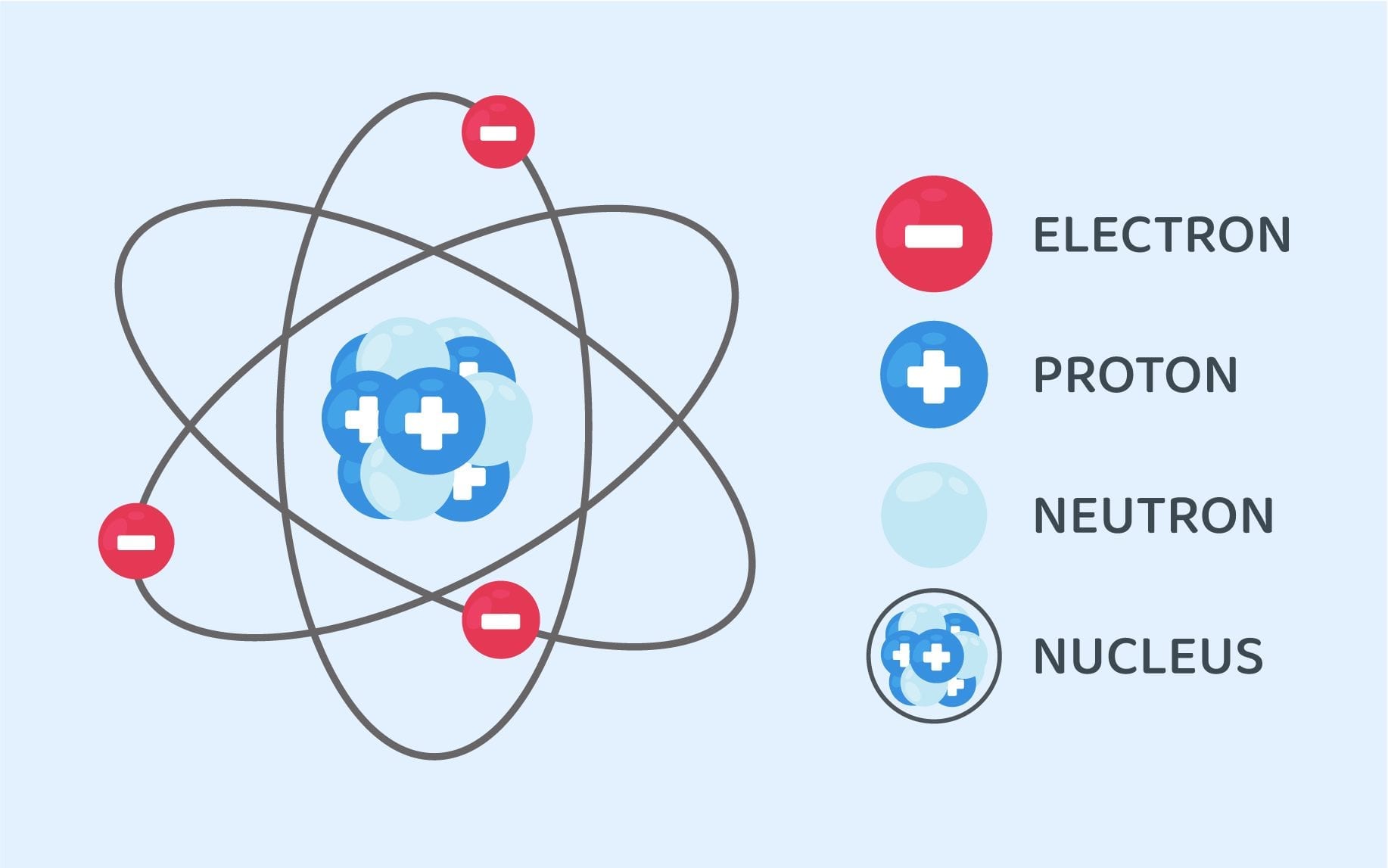
Structure Of An Atom Class 9 Science Notes Leverage Edu

4 2 Occurrence Preparation And Properties Of Transition Metals And Their Compounds Inorganic Chemistry For Chemical Engineers

Chemistry Chapter 9 Assessment Answers

Anatomy02lecturenotesradio 1 Chapter 2 Notes Basic Chemistry Matter And Energy Stephens Fall 2015 Matter Anything That Occupies Space Course Hero

Example 2 Show That 1 7 4 2 1 1 4 4 Examples

Find The Equation Of The Circle Of Radius 5 Cm Esaral

Rs Aggarwal Solutions For Class 9 Maths Chapter 14 Indcareer Schools

Rs Aggarwal Solutions For Class 9 Chapter 2 Ex 2e Pw
Article Writing Class 9 Format Topics Examples Samples

Viraf J Dalal Solutions For Class 9 Simplified Icse Chemistry Chapter 7 Study Of Gas Laws Latest Edition Shaalaa Com